| PLANT CULTURE
(Greenhouse/Growth Chamber) |
|
| Container.............. | Seedling flats subdivided into compartments, with bottom drainage holes; flats are placed in a water reservoir (a flat without holes). Watertight tubs with drain holes that can be plugged/unplugged to allow drainage may also be used. |
| Media................... | Autoclaved sand, porous soil mix, or vermiculite. |
| Temp/Light............ | 20 to 24 C; 12 to 16 hour day length |
| No. of plants.......... | 50 to 70 per replication |
| No. of Reps.......... | 4 minimum |
INOCULUM SOURCE
| Standard Isolates.. | MF-1 (Race 1) NC-1 (Race 2) |
| Storage................. | Oatmeal or corn meal agar |
| Temperature.......... | 4-12 C. For 4 C storage, use constant temperature incubator only, not self-defrosting refrigerator. |
INOCULATION PROCEDURE
| Age of Plant.......... | 5 to 6 days (when cotyledons are fully expanded) |
| Type of Inoc.......... | Zoospore suspension or comminuted mycelium |
| Production............. | Zoospores produced by the method of Mitchell and Yang (3); or one, 1 week old corn meal agar cultures are blended in 1 L distilled water |
| Concentration........ | 100 to 1000 zoospores or 1 mL comminuted mycelium per seedling |
| Method................. | Add water to the surrounding reservoir to saturate the entire root zone, then drench inoculum over the seedlings into the upper root zone |
INCUBATION
| Location................ | Environmentally controlled chamber or greenhouse Plant |
| Counts.................. | Count at full emergence (7 to 8 days after seeding) |
| Culture.................. | Maintain flooded conditions for 5 days; application of a complete nutrient solution to the water reservoir 7 days after inoculation aids in separation of plant reactions. |
| Age at Rating........ | 10 to 14 days after zoospore inoculation; 5 weeks after inoculation with mycelium |
RATING
| Percent resistant plants is the total of classes of 1 and 2. |
| 1 Resistant............ | No necrosis of roots and hypocotyls |
| 2 Resistant............ | Slight necrosis of roots and hypocotyls |
| 3 Susceptible........ | Necrosis of roots and lower hypocotyl, slight chlorosis of cotyledons, and moderate stunting of stem(s) |
| 4 Susceptible........ | Extensive necrosis of roots, hypocotyls and cotyledons, and severe stunting of stem(s) |
| 5 Susceptible........ | Dead seedling |
CHECK CULTIVARS
| Approximate Expected Resistance(%) | Acceptable Range of (Reaction (%) | |
| Race 1 | ||
| Resistant WAPH-1 (1) |
50 | 35-60 |
| Susceptible
Saranac |
2 | 0-5 |
| Race 2 | ||
| Resistant WAPH-5 |
50 | 35-60 |
| Susceptible Saranac |
2 | 0-5 |
| WAPH-1 | 2 | 0-5 |
| Values for resistant standards are percent of total plants in classes 1 and 2. Both Saranac and WAPH-1 must be used as race 2 susceptible checks. | ||||||||||
|
Aphanomyces
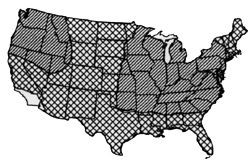
Root Rot (Aphanomyces euteiches Drechs) A. euteiches, has been reported throughout North America, Europe, Australia, and New Zealand. The distribution of alfalfa strains per se has not been exhaustively studied. However, race 1 alfalfa strains have been detected in Idaho, Illinois, Indiana, Iowa, Kentucky, Maryland, Michigan, Minnesota, Mississippi, Nevada, New York North Carolina, Ohio, Oklahoma, Pennsylvania, Tennessee, Virginia, Wisconsin, and Ontario and Quebec, Canada. Race 2 alfalfa strains have been confirmed in Idaho, Maryland, Minnesota, North Carolina, Iowa, Tennessee, Virginia and Wisconsin (2). Aphanomyces can cause severe stunting and death of seedlings, and can cause a chronic disease of lateral roots of established plants. It frequently is recovered from fields where Phytophthora root rot and Pythium damping off are found. A. euteiches and Phytophthora medicaginis may cause a root disease complex. Aphanomyces root rot is favored by warm, saturated soil conditions. SOURCE OF INOCULUM AND SCIENTIST WITH EXPERTISE
CORRELATION TO FIELD REACTION There is a good correlation between results of this test and visual root scores, plant vigor and forage yield in naturally infested fields (4). RACES There are two recognized races of A. euteiches. Isolates are known which may belong to additional races, as yet not defined. PLANT GROWTH OPTIONS AND
RANGE OF CONDITIONS
HELPFUL INFORMATION
ALTERNATIVE METHODS
REFERENCES Acknowledgments: Thanks to John Edmonds, Jay Sandman, Sam Stratton, and Mike Velde for supplying additional data used in the development of this test. |